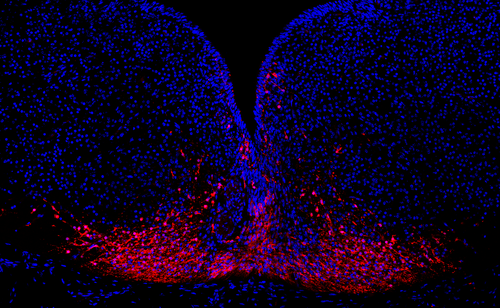
Research activities are focused towards the functional characterization of a group of type-1 receptors denoted the Vps10p-domain family, or so-called sortlins, that comprises sortilin, SorLA, and SorCS-1, -2, and -3. The receptors are enriched in neurons where they mediate trafficking and signaling of a vast number of ligands such as neurotrophic factors along with their cognate receptors, neurotransmitter receptors, APP, and progranulin. Among many activities, the receptors regulate neuronal cell fate, differentiation, innervation, synaptic plasticity, and learning and memory. Key goals of the Nykjaer lab is to understand their functions in the heathy brain, dissect out their mode of actions, investigate how genetic variation contributes to disease development - in particular of neuropsychiatric disorders and memory impairment -, and to evaluate their potential as drug targets.
Methodologies include transgenic mice and zebrafish, a broad repertoire of molecular, cellular and genetic and viral tools, transcriptomics and (phospho)proteomics, neuroembryology, mouse behavior, electrophysiology and advanced imaging including high-resolution microscopy. In vivo fiber photometry and mesoscale single unit recordings are currently being implemented.
The Nykjær group currently has projects available for Master students and postdocs within the following research areas.
Functions of the sortilin receptor family in health and disease:
Please contact Group Leader Anders Nykjær directly, if interested.