Taro Kitazawa appointed new Group Leader at DANDRITE
Taro Kitazawa will start his research activities on molecular basis of neuroplasticity in memory formation in the summer 2022.

Dr. Taro Kitazawa did his PhD studies at the Department of Medicine at University of Tokyo in Japan, and then postdoctoral research at the Friedrich Miescher Institute for Biomedical Research (FMI) in Switzerland.
Dr. Taro Kitazawa’s research focus is on understanding the epigenetic and transcriptional basis of neuroplasticity that underlies memory engram plasticity using rodents as model organism.
Dr. Taro Kitazawa’s research activities complement the expertise and research programmes of the other current research groups at DANDRITE, where first five Group Leaders recruited at DANDRITE are focusing on embryonic and reprogrammed stem cells in Parkinson disease (Dr. Mark Denham), the brain circuitries and behavior in Drosophila (Dr. Anne von Philipsborn), the structure, function and development of neural circuits in the visual system (Dr. Keisuke Yonehara), the genetic and neuronal circuit mechanisms that underlie effort based decision-making in flies, rodents and humans (Dr. Duda Kvitsiani) and the plasticity at the synaptic and circuit levels underlying learning and memory formation (Dr. Sadegh Nabavi).
The DANDRITE core groups are focusing on structural biology of membrane transporters, receptors and biomembranes (Prof. Poul Nissen), sorting/trafficking, trans-synaptic communication and signaling in nerve cells as mediated by sortilin receptors and neurotrophic factors and studied in transgenic animal models (Prof. Anders Nykjær) and molecular cell biology of intracellular signaling networks with focus on Parkinson disease and dementia (Prof. Poul Henning Jensen).
Furthermore, the DANDRITE researchers engage in many collaborations at Aarhus University, Aarhus University Hospital, and the Danish neuroscience community.
Detailed description of Dr. Taro Kitazawa’s research
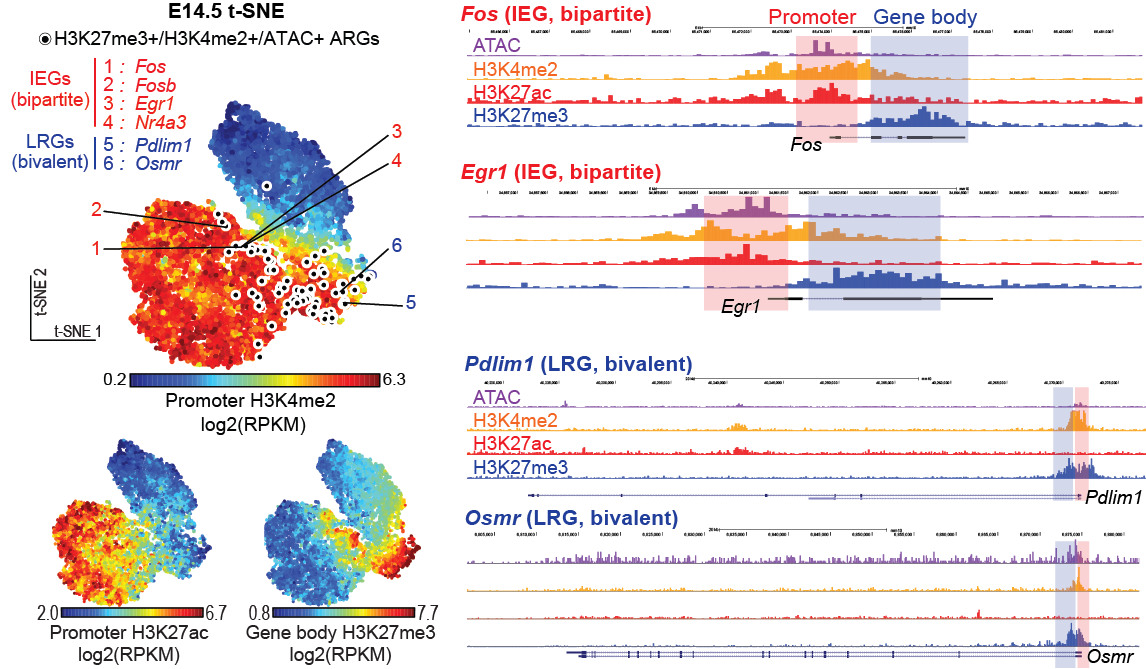
Neuroplasticity underlies learning and memory formation, which allows for accumulation of knowledge. Abnormalities can be associated with various disorders including dementia and post-traumatic stress disorder (PTSD). There is an emerging view that a sparse ensemble of neurons, termed as memory engram cells, store specific memories. Memory engram cells are defined as neurons that are transiently activated and express immediate early genes (IEGs) during a learning experience and have undergone physical changes that can be reactivated upon memory recall experience. Importantly, artificial activation and silencing of engram cells result in artificial memory retrieval and deficit, respectively, and open for research approaches to unveil the mechanisms.
Revealing the molecular basis of neuroplasticity in engram cell formation is considered one of the central issues of memory research.
In a recent study, Dr. Taro Kitazawa and his colleagues at the Friedrich Miescher Institute for Biomedical Research (FMI) in Switzerland revealed a novel epigenetic and transcriptional mechanism regulating neuronal activity-dependent IEGs activation during somatosensory neuron maturation (Kitazawa et al., Nature Genetics, 2021, Figure).
In the DANDRITE, Dr. Taro Kitazawa will take an advantage of his research expertise and further investigate how neuronal activity-dependent gene regulation underlies memory engram cell plasticity.
In his efforts to address this issue, Dr. Taro Kitazawa will carry out epigenetic and transcriptional profiling of memory engram cells using state-of-the-art high throughput sequencing genomics technologies. Engram cells will be isolated from the mouse hippocampus or neocortical regions, which store recent (in the order of days) or remote (in the orders of weeks and months) memory, respectively.
With this approach, he will firstly reveal the molecular underpinning of engram cell state shifts upon memory transfer from the hippocampus to the neocortical regions, which has been referred to as the systems memory consolidation. Furthermore, he will also focus on the heterogeneity of memory engram cells, and aim to reveal how a subset of engram cells can be functionally more relevant as compared with other subsets of engram cells. Notably, to efficiently answer these questions, he will even develop a novel genomics technology which enables whole genome history tracing, and overcome critical limitations of existing snapshot-type sequencing technologies.
Dr. Taro Kitazawa’s work will be also supported by start-up grant of the European Research Council (ERC), which provides funding for ground-breaking international research projects of scientific excellence in all subjects and fields of research.
The Danish Research Institute of Translational Neuroscience – DANDRITE is the Danish node of the Nordic EMBL Partnership for Molecular Medicine and therefore adhere to the EMBL model for international recruitment.
DANDRITE is established with support from the Lundbeck Foundation at Aarhus University. The research scope is on molecular mechanisms underlying intra- and intercellular signaling networks that govern neuronal functionality and circuitry, how these mechanisms define behaviour and are altered in neurological and psychiatric disorders.
DANDRITE is embedded in the vibrant NeuroCampus research community at Aarhus University and Aarhus University Hospital and encompasses genetics, molecular and clinical medicine, bioimaging, cell and animal modeling, structural biology and nanoscience, and with long-standing traditions in membrane proteins and neuroscience.
