New publication from Nissen lab: “A non-helical region in transmembrane helix 6 of hydrophobic amino acid transporter MhsT mediates substrate recognition”
The publication reports on the studies of the amino acid transporter MhsT and provides important clues to moelcular mechanisms of specificity yet as well with a broad pool of substrates that range from valine to tryptophan. Previous and current PhD students Dorota Focht and Caroline Neumann, and previous postdoc Joseph Lyons from the Nissen lab are first authors on the study that involved crystal structure determination of six different substrate-bound complexes of MhsT and transport kinetics studies in a cellular model and proteoliposomes of MhsT function.
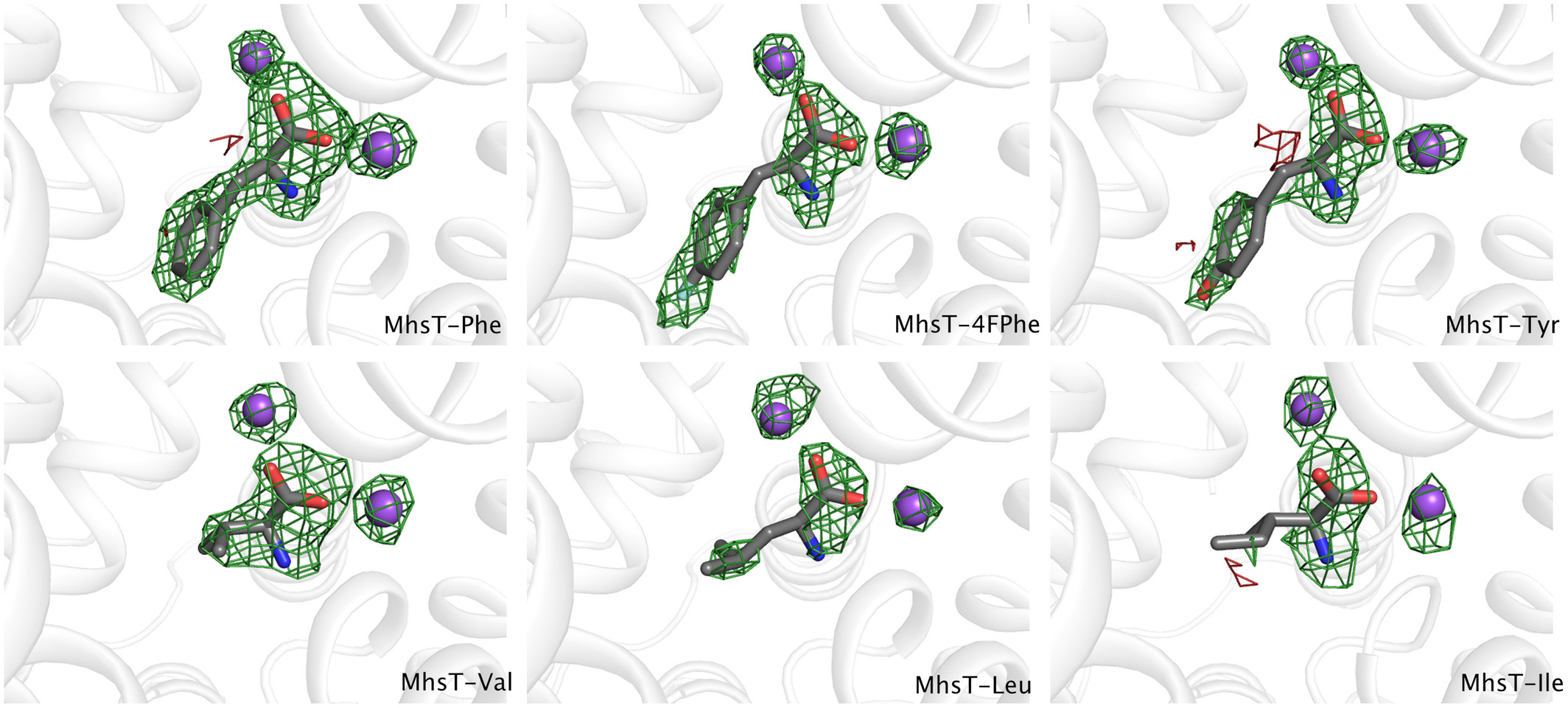
The publication “A non-helical region in transmembrane helix 6 of hydrophobic amino acid transporter MhsT mediates substrate recognition” was published in EMBO Journals.
Link to the article: https://doi.org/10.15252/embj.2020105164
MhsT is homologous to the human SLC6 transporter family including for example the serotonin transporter SERT and the glycine transporter GlyT1, which has just been published in Nature, coming out in print today March 25th, 2021 (see a previous news release here: Structure determination of the glycine transporter GlyT1 opens new avenues in development of psychiatric drugs).
The publication “Structural insights into the inhibition of glycine reuptake” was published in Nature.
Link to article: https://doi.org/10.1038/s41586-021-03274-z)
